Getting the vaccines to their destination in good condition is paramount for a successful immunization campaign. When exposed to an elevated temperature for too long, they lose their potency. For patients with chronic diseases, taking a compromised dosage can be dangerous. Preventing cold chain failures through remote monitoring systems is vital.
It can be challenging to know the difference between a compromised product and a safe one. Pharmaceutical and vaccine providers must take measures to ensure products are stored correctly.
The cold chain is the process for keeping medicines at a cold temperature, from manufacturing through distribution and storage to end-use. The cold chain can be broken due to many reasons.
Preventing cold chain failures, logistic providers use remote monitoring systems. Alerts are sent when temperatures deviate from the prescribed thresholds, threatening medicines.
Equipment failure
Knowing about equipment malfunctions or breakdowns is essential to protecting medical products. Refrigerators maintain a constant temperature to stop spoilage to the medical products. Preventative maintenance minimizes expensive repairs. But what happens if an issue occurs between the scheduled maintenance period?
To protect the inventory sensors and remote monitoring systems, check the temperature—the monitoring system checks for equipment failure, including power and temperature fluctuations.
These systems can act as data loggers. Vaccines for Children (VFC) providers must have such sensors installed. They are recommended for any facility that stores refrigerated or frozen pharmaceutical products. They store a record of all temperature data that can be downloaded at any time to show compliance. This data identifies trends in environmental conditions and detect problems before becoming critical.
Reasons for breaking the cold chain
Coolant/Insulation Failure.
Temperature-controlled containers with passive cooling rely on a coolant part of the packaging. The packages are sealed and insulated. However, the packaging is not infallible. Improper handling could damage the packaging, rendering the insulation and coolant compromised.
Poor Air Circulation.
Temperature-controlled environments are relying on active cooling need proper air circulation for optimum cooling. Clogged vents or hotspots in temperature-controlled zones are significant problems in cold chains. By the time it is detected, it is usually too late to reverse the damage.
Insufficient Redundancy.
Breakdowns happen, as do delays. Cold chains accommodate these events to a degree. But there should be redundancy or backup systems in place.
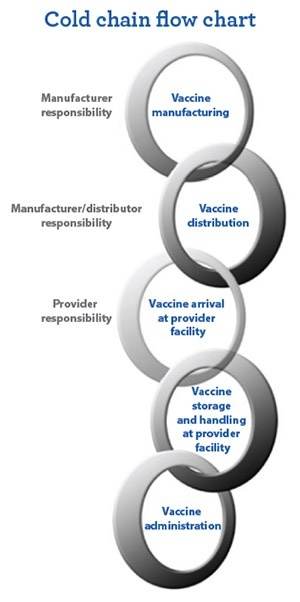
Photo credit: blog.smartsense.co
Lack of Uniform Standards or Infrastructure
Differences in Technology, infrastructure, and cold chain standards such as:
- Lack of adequate temperature-controlled warehouses
- non-uniform cooling standards across intermediate storage facilities.
It makes it difficult for individuals running global operations to guarantee end-to-end integrity.
Power failure
Power Outages. In some areas, mainline power can be unreliable. Trucks or other modes of transport with no redundant backups are vulnerable systems. To ensure vaccines and medicines are stored correctly, maintain a reliable power supply. Power outages can cause a break in the cold chain, resulting in the loss of medical products.
Remote monitoring systems notify the moment there is a change in power status. The personnel responsible will receive an alert via text message, email, or phone call. Additionally, a battery backup system will provide 8 hours of continuous monitoring. Remote monitoring systems give plenty of time to address the problem before the inventory is compromised.
Human error
Human error is another leading factor in breaking the cold chain failure. Human error occurred when refrigerators and freezers were left unmonitored. Human error happens because the staff forgot to log temperatures or a door left open.
Remote monitoring systems minimize the risk of human error. It ensures that temperatures are within recommended ranges. Also, enable corrective action when temperatures deviate. And alarms when temperatures change or the refrigerator door is left open. These features are useful if the facility is unattended for an extended time.
Vaccines and pharmaceutical products are expensive and sometimes slow to replace. Remote monitoring systems are 24/7 and provide an audit trail for proving compliance.

Distribution/Delivery Cold Chain Risk
Unlike fixed temperature-controlled warehouses, mobile cold storage has more factors to consider. There is an increased risk of equipment breakdowns and packaging failures—also, difficulties in saving stranded shipments due to studies in remote locations.
Theft/Pilferage
Cargo theft is a persistent problem in the supply chain across the world. Whether regular pilferage and hijack of a full truckload. Cold chain trucks are now high-value targets for cargo pirates.

Risks in Cold Chain Management and Preventing Cold Chain Failures
Despite moves to mitigate risks, cold chains still fail and expose products to the risk.
Global community companies are standardizing reliable cold chains that guarantee product’s quality. No matter the destination is, the products must be in good condition and safe for consumption.
The damage does not just hit the company’s profit base. It has consequences for global food security and healthcare assurance as well.
Over one-third of the world’s food is lost to spoilage each year due to inadequate temperature control techniques during transit. It not only affects the availability of food sources, but also medical treatments in the world.
It is vital to understand the most significant risks to their cold chains but also how to solve them.
Preventing Cold Chain Failures
Cold chain interruptions will always be there, despite the company’s most diligent deterrents. Ensuring 100 percent cold chain reliability is possible. However, it is challenging to balance the cost of cold chain risk management to reduce costs. But supply chain professionals today have the advantage their predecessors did not, the Internet of Thing. IoT combined low-cost sensors and real-time shipment monitoring to help cold chain management.
Proper end-to-end IoT cold chain monitoring solution gives shipping managers and quality control personnel what needs to ensure cold chain reliability:
1.) Sense Data in Real-time
Knowing the moment about a temperature spike and which cargo can potentially be affected is valuable information.
GPS and sensors in IoT devices help determine where the temperature anomaly is happening and what possibly triggered it.
2.) Make Sense of Data
Another advantage to continuous monitoring is aggregated data. Useful logistics data is a source of predictive and real-time actionable insight.
There are variables used to recommend more efficient routes, such as:
- Window of delivery
- Route congestion
- Possible deviations
- Fuel consumption
- Remaining coolant
Using these have a positive impact than sweeping procedural changes rife with risk.
3.) Act on Insights Through Timely Intervention
In delayed shipments, real-time monitoring would notify with enough time to take action. Therefore, dispatching a recovery or refit team to resolve the issue.
Intervention cost is lower than overcompensating cold chain risk mitigation tactics. It also allows a leaner and reliable cold chain.
Global logistics operation is a good idea for outsourcing critical tasks to the response center. Outsourced logistics control towers are catching on with logistics operations that handle large operations in the world.
Doubling up preventive measures is not the solution to reduce cold chain logistics risk. Decision making needs reliable data gathering, constant connectivity, and advanced analytics. For an end-to-end. Cold chain monitoring solution.
Pharmaceutical companies’ biggest challenge today is to maintain cold chain refrigeration for vaccines. A misconception among healthcare professionals that “colder the better” for vaccine storage. Resulting in the loss of money and time spent implementing revaccination programs.
Unfortunately, U.S. agencies do not require rigorous regulations for monitoring vaccine temperatures. That is why stakeholders should be educated about the consequences of inconsistent temperatures. Only then can effective monitoring plans be put in place throughout the cold chain.

Why Is the Cold Chain So Important?
Cold chain is the preservation of vaccines from manufacturers to delivery to health care facilities. Stakeholders should monitor the vaccine temperature range at each stop. Failing to monitor vaccine temperatures accurately may result in:
- Formula degradation
- Loss of potency
- Waste of product and money
- Substandard immunologic responses
- Need to re-immunize patients
Conclusions:
Studies have shown how the cold chain is frequently unreliable in developing nations—especially regions where ambient temperatures severely exceed more than 8°C (46.4°F). For example, a 2015 survey from Cameroon found that only 50% of vaccines needing refrigeration were regularly monitored in 81% of health care facilities.
Equally problematic, up to 35% of vaccines worldwide are exposed to freezing temperatures. Worse, education programs to improve storage conditions for vaccines are minimal. In one study from India:
Only 26% of all clinics had written instructions for handling vaccines.
Only 60% kept up-to-date temperature logs.
Fixing Breaks in the Cold Chain Saves Time & Money
The media reported discarding of Frozen vaccines, and patients needed revaccination. No wonder vaccine wastage and revaccination are expensive. A U.S. survey suggested that 1%–5% of vaccines are wasted, potentially costing up to $31 million. Most of these incidences can be solved by fixing breaks in the cold chain.
Reference links:
https://blog.smartsense.co/vaccine-storage-the-importance-of-maintaining-the-cold-chain