Why Cold Chain Monitoring is Critical in the Delivery of Vaccines
Vaccine monitoring throughout the cold chain until final delivery is essential. Vaccines are a key component in our health care system. One of the most cost-effective and efficient public health strategies is immunization. The end of many disease outbreaks can be directly attributed to the development of their respective vaccines. They have an immense societal contribution, which is why it is crucial that their delivery is handled with the utmost care to ensure that the vaccine’s integrity is maintained all throughout its transport. The effectiveness of vaccines heavily relies on proper vaccine storage conditions. Vaccines are time- and temperature-sensitive. They are transported through a cold chain to ensure that their specific temperature needs are met at all times.
In a pharmaceutical context, the cold chain refers to the process of maintaining consistent temperatures for vaccines from manufacture through delivery to the healthcare facility. The cold chain starts from the cold storage unit located at the manufacturing plant and includes the transportation and delivery of the vaccine to the proper cold storage facility. It ends with the successful administration of the vaccine to the patient. Preparing the cold chain for vaccines is a massive challenge for vaccines. At a glance, it may seem that a colder cold chain would be better for vaccines. However, over-chilling vaccines may result in a substantial financial loss and a counterproductive implementation of revaccination programs due to the infectivity of initially delivered vaccines. Aside from that, failure to properly deliver the vaccines may also lead to a patient’s loss of confidence in the vaccination program.
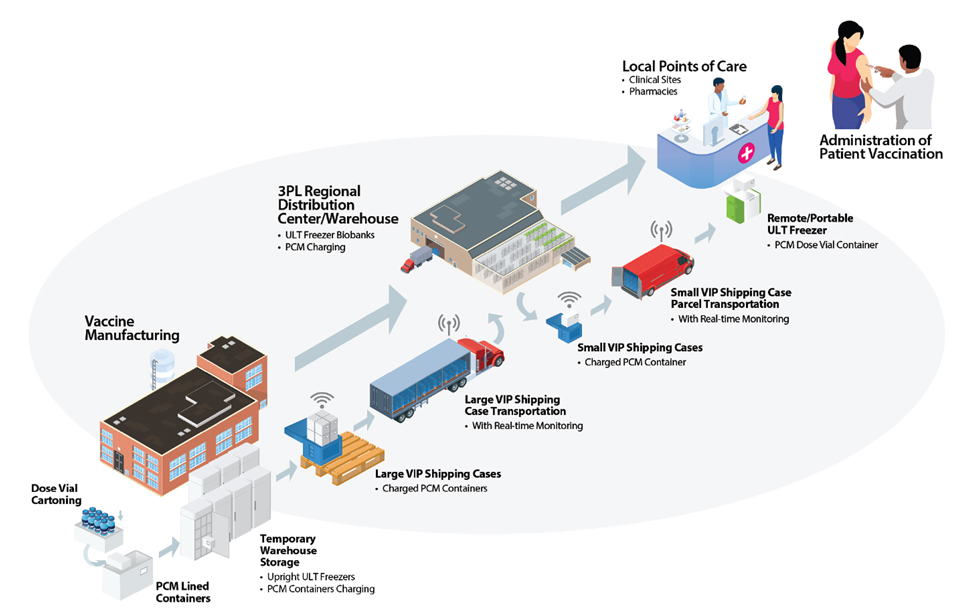
Cold chain staff should monitor the vaccines at each stop of the chain to ensure that the vaccine’s temperature is kept at the optimum range (2°C and 8°C or 36°F and 46°F). Meanwhile, freezers should give a temperature between -50°C and -15°C (-58°F and +5°F). Refrigerators and freezers should be set at the midpoint temperature to decrease the possibility of a temperature excursion occurring.
Ineffective vaccines cannot be used and need to be disposed of immediately. If a vaccine is not monitored accurately, it may result in loss of potency, degraded formula, product waste, financial loss, and the need to re-immunize patients.
How Temperatures are Monitored
Routine monitoring activities allow the concerned personnel to take immediate action in the event of a temperature excursion. If the temperature monitoring plan is not implemented correctly, it might pose problems for the vaccine cold chain. Vaccine storage units often have reliable Temperature Monitoring Devices (TMD). In order to properly monitor the temperatures, the
Centers for Disease Control and Prevention (CDC) prescribes the use of a digital data logger (DDL), which serves as the recording and continuous monitoring device, set to record at 30-minute intervals. DDLs often use buffered temperature probes to measure temperature. Buffered probes measure actual vaccine temperatures more closely compared to standard temperatures. DDLs provide data on how long a unit has fallen outside the allowable temperature range. To ensure accuracy, DDL should have a valid Certificate of Calibration testing.
Special software is used to extract data that the DDL has recorded for user review. This software also enables the user to control the frequency of temperature readings. Each vaccine storage and emergency transport unit should have a TMD.
Staff should check and record the minimum and maximum storage unit temperatures at the beginning of each workday. If the TMD being used does not display the minimum and maximum temperatures, then the current temperature should be checked at the start and end of the workday.
A log sheet should be placed at the storage unit door to monitor the temperature. The following details should be taken note of:
- Date
- Time
- Name of personnel who recorded the temperature
- Actions that were taken in the event of a temperature excursion
- Minimum/maximum temperature (or current temperature if using a TMD device that does not show the minimum and maximum temperatures)
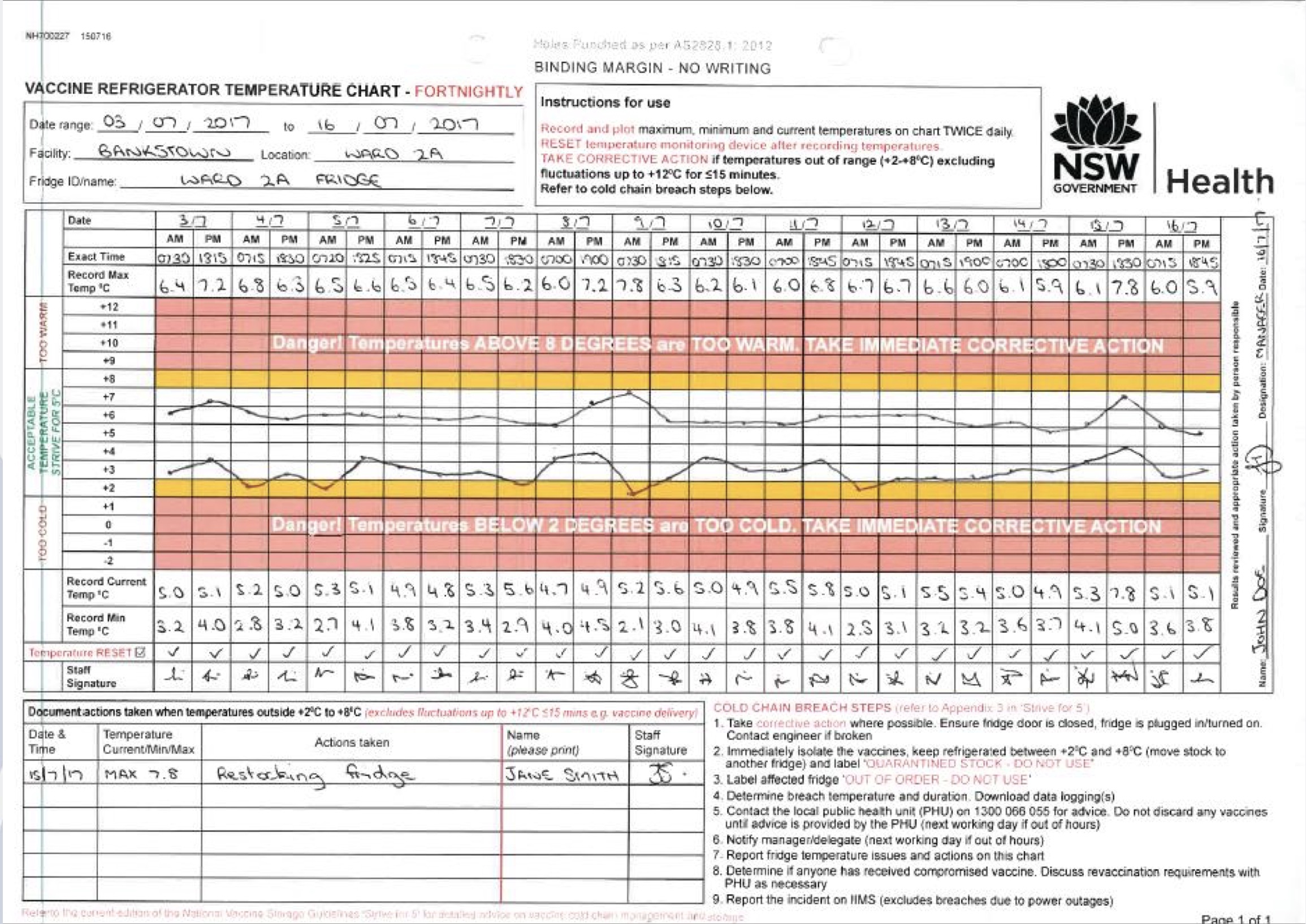
Unit doors should always be tightly closed. It is best to review storage unit temperature readings per week regarding the changes in the temperature trends that might require immediate action like replacing equipment or needed adjustments regarding the unit temperature. Temperature data should be stored for three years to better study reoccurring temperature patterns.
Some temperature monitoring devices are not recommended by CDC since they only display the temperature at the exact moment they are checked. These devices also tend to fail in the detection of the temperature outside the allowable range.
The following devices are not recommended by the CDC:
- Liquid vial Bimetal stem TMDs
- TMDs for food
- TMDs without a valid Certificate of Calibration Testing
- Alcohol or mercury thermometers
- Chart recorders
Devices sold in appliance and hardware stores are often intended to monitor temperatures for household food storage, which is why they are not calibrated and tend to be inaccurate when used for monitoring vaccine delivery. Devices like these highly increase the risk of damaging vaccines.
Case Study – Monitoring Vaccine Delivery
Keeping in mind standard World Health Organization (WHO) study protocols, a vaccine cold chain monitoring study was conducted by an Elsevier team to record possible problems and determine the necessary control measures. Several TMDs were used to assess if they are user-friendly and record the staff’s attitude towards them. During the study, all facilities followed their routine practice in terms of storage. The temperature monitoring devices featured for the study were read by staff on arrival and dispatch to every facility.
Most of the time, temperatures were kept at the recommended range of 20°C – 8 °C. A wider temperature variation was observed when the products were moved from the laboratory to service points. The vaccines encountered temperature excursions at times; however, a shake test with the vaccines revealed no freeze damage. Most of the staff believe that a shake test is insufficient in determining a freezing event. 91% of the staff stated that they did not have the appropriate tools to see whether a vaccine had been damaged due to temperature excursions. They were informed of the cold chain problem areas through this study, and they believed that including the appropriate TMDs would enhance their current cold chain operations. The researchers recommended an expanded monitoring program with unit-level freeze and heat indicators. Because the indicators provide a visual signal, it would be easy to see whether a temperature excursion was experienced at some point in the cold chain. WHO recommended that a 30-day electronic refrigerator temperature logger should be installed in service point refrigerators to ensure that temperatures would be monitored even during weekends and holidays.
The use of these tools would help health workers to make informed decisions that will prevent vaccine wastage due to temperature-damaged vaccines. Properly monitoring vaccines would ultimately increase the overall effectiveness of vaccination programs.
Emergency Response
All staff should be informed of the emergency response measures in case of a cold chain equipment breakdown, transport emergency, power supply failure, and various other situations that may endanger vaccines. Managers should formulate contingency plans for the facility and equipment that would contain the required actions needed to be undertaken in response to common emergencies.
Certain key elements should be factored in when developing a contingency plan
- In case of a temperature excursion, the basic emergency safe storage rule should be observed, which is to place the affected vaccines in a 2–8 °C environment in the soonest possibly time
- Alternative locations should be identified where vaccines can be securely stored or where ice is readily accessible at short notice.
- Emergency contact details should be easily available
- Initial and follow-up actions should be laid out that can be implemented inside and outside working hours
- In order to guarantee the relevance of the plan, a yearly review should be conducted.
In case of an emergency situation, the source of the problem should be immediately located, identified, and rectified. The vaccine coordinator must report the problem to a supervisor as soon as possible. If the issue cannot be resolved immediately, the vaccines should be protected by implementing the basic safe-storage rule. Vaccines should not remain in a nonfunctioning unit for a long time period.
The staff should conduct the facility’s standard operating procedures for temperature excursions. Temperature monitoring devices should be checked, and the unit temperature should be adjusted as needed. Once the situation has been mitigated; the vaccines should then be studied if they have been damaged through the following:
- Heat exposure: inspect the VM status of the vaccines for possible heat damages, especially the most heat-sensitive ones.
- Freeze exposure: perform the shake test with freeze-sensitive vaccines.
If there is suspected vaccine damage, the vaccines in question should be labeled as damaged or potentially damaged and immediately isolated at the required storage temperature until a final decision has been made regarding its disposal or use. If there is confirmed damage, the vaccines should be removed from the cold chain. The emergency event should be recorded for documentation purposes. The necessary reports should be made so the supervisor will be well informed to make a decision regarding the next steps that should be taken.
Remote Vaccine Delivery Monitoring
AKCP provides complete monitoring of the vaccine delivery system for the pharmaceutical cold chain. In addition to vaccines, there are many temperature-sensitive drugs that also require monitoring. We can monitor an individual medical refrigerator, warehouse, multiple sites, and vaccines in transport. With AKCPro Pharma-Mon Server you can monitor inventory, expiration dates and generate Digital signs reports in accordance with FDA 21 CFR Part 11.




